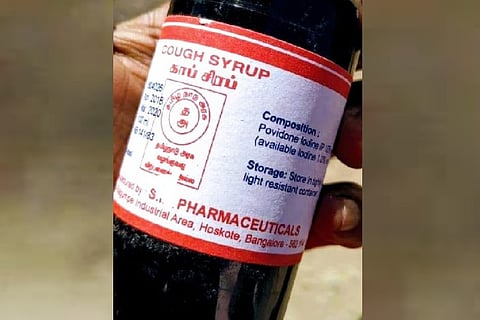

Government hospitals and drugstores in Tamil Nadu were in a frenzy on Monday as a picture of an antiseptic labelled as cough syrup along with the logo of the Government of Tamil Nadu went viral on social media.
According to news reports, the cough syrup which should have compounds like codeine and dextromethorphan hydrobromide was labelled as Povidone Iodine, which is used as an anti-microbial solution by hospitals before surgeries.
As the news of mislabelling spread, the state health department ordered a probe into the matter. Tamil Nadu Medical Services Corporation (TNMSC) Managing Director P Umanath attributed the confusion to errors in labelling, including the logos. He told Times of India that the manufacturer of the item was the supplier for both Tamil Nadu and Rajasthan and that he had mislabelled the iodine solution to be supplied to Rajasthan with the logo of Tamil Nadu. Umanath also stated that the manufacturer had already recalled the mislabelled medicines from Rajasthan and that the Rajasthan government might take action on him for the mix-up. He also said that the TNMSC will impose a fine on the manufacturer for misusing the state government’s logo.
TNMSC stopped buying combination drugs since September 2018 after the Central government banned them due to increasing antibiotic resistance on account of multiple drugs being consumed by the people. However, a pharmacist told New Indian Express that the Tamil Nadu government still supplies combination cough syrups.
The Central government last week banned 80 more Fixed-Dose Combination drugs including painkillers and antibiotics. This is over and above the 325 drugs banned by the Union Health ministry last September. These drugs have been banned on the recommendation of Drugs Technical Advisory Board (DTAB), which tests the safety of the medicines, said reports. However, drug manufacturers have approached the Supreme Court against the ban and the case is pending.